 |
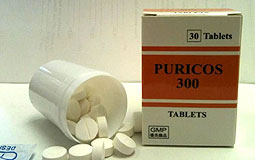
Drug alert: The Department of Health has ordered the recall of Puricos 300 tablets. |
|
The Department of Health has ordered licensed drug wholesaler Julius Chen & Co (HK) to recall Puricos 300 tablets (HK-31027) which have been packaged without a manufacturing licence.
The drug was made in South Africa and is used for treating gout and hyperuricaemia. It can be sold in pharmacies. Some of the product has been sold to private doctors and pharmacies.
The company illegally stored medicines and was involved in illegal packaging of pharmaceutical products without a manufacturing licence.
Another illegally packaged product, Nutribes Folic Acid tablets, which the company imported for re-export to Macau, were also found in the investigation.
Healthcare professionals and retailers should stop supplying the product to clients. People who have bought it should stop using it and seek medical advice if they feel unwell.
The company has set up a hotline - 2487 1301 - to answer enquiries. It has suspended its wholesale activities to facilitate the department's investigation.
Pharmaceutical products imported by drug wholesalers must be packaged by local licensed Good Manufacturing Practices manufacturers before distribution to users and retail outlets.
Go To Top
|



