Capsule recall:
The Department of Health ordered the recall Hong Fu Ganmaoging Capsules...
Capsule recall:
The Department of Health ordered the recall Hong Fu Ganmaoging Capsules...
ArticleSummary1
... and G.L. Savior.
Tainted medicines recalled
May 25, 2012
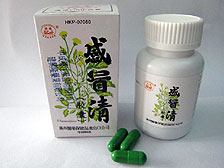
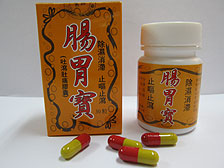
The Department of Health today instructed two wholesalers, Fu Yuen Trading Company and Kian Hin Leong Medicine Company, to recall two proprietary Chinese medicines - G.L. Savior and Hong Fu Ganmaoging Capsules.
The wholesalers have declared the products were manufactured by Xinchang Chengxin Capsule Company and Zhejiang Kangnuo Capsule Company.
They have been found by the State Food & Drug Administration to have excessive chromium levels. Both wholesalers could not provide valid laboratory test reports to prove the chromium levels of their medicines were within the recommended limit.
The department investigated and obtained samples of the medicines from both wholesalers for tests. The Government Laboratory found the capsule of a sample of G.L. Savior contained chromium at 44ppm, while that of Hong Fu Ganmaoging Capsules contained chromium at about 10ppm. Both exceeded the recommended limit of 2ppm in the Pharmacopoeia of China.
People can call Fu Yuen's hotline at 2788 1082 and Kian Hin Leong's hotline at 2548 9551 for enquiries.
Those who have purchased the medicines should stop using them immediately and see a doctor if they feel unwell.