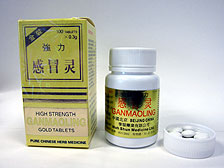
Arsenic alert:
The Department of Health has ordered the recall of Ganmaoling tablets batch number 801128A1.
Chinese medicine recalled
June 24, 2011
The Department of Health today ordered the recall of Ganmaoling tablets batch number 801128A1, which has been found to contain an excessive level of arsenic.
The drug was manufactured on the Mainland and imported by Wah Shun Medicine December 2009.
Arsenic can harm the liver, kidney and heart.
Users of the product feeling unwell should seek medical advice. The department has not received any reports of illness associated with the product so far.
Mainland authorities have been informed of the recall.