 |
| Safety alert: People should not take this slimming product which contains undeclared drug ingredients. |
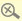
|
The Department of Health urges people not to buy or take a slimming product in the form of green capsules as they contain undeclared drug ingredient sibutramine.
The department received a report from a private hospital about a poisoning case involving a 56-year-old woman who had taken the product bought in Shenzhen.
She developed insomnia, poor appetite and thirst. She came down with with palpitations, sweating, restlessness and shortness of breath on July 9 and was admitted to hospital. She was discharged today after her condition improved.
Sibutramine can increase blood pressure and heart rate, and cause mental disturbance and possible convulsions.
Products with sibutramine must be registered, sold on a doctor's prescription and dispensed under a pharmacist's supervision in Hong Kong.
Go To Top
|



