63 anti-epidemic projects get funding
Sixty-three projects have been approved under the Public Sector Trial Scheme to combat the COVID-19 epidemic with total funding of over $102 million, the Innovation & Technology Commission announced today.
Trials of the projects' research and development (R&D) outcomes have commenced progressively in the public sector, the commission added.
Lasting from March 9 to April 10, the special call under the trial scheme received 332 applications. The approved projects come from local universities, R&D centres, designated local public research institutes and technology companies conducting R&D activities in Hong Kong.
They fall under a number of categories including COVID-19 virus detection or diagnosis methods, masks and other protective equipment, disinfection equipment and products, body temperature checking devices and virus transmission tracking devices.
Fifty-seven public organisations are involved in the trials of the approved projects with Fung Kai Care & Attention Home for the Elderly being one of them.
It participated in the Centralized Nano Bubble System for Surface Cleaning & Sanitization trial project that splits ozone into nano bubbles.
The seniors' care home noted that apart from being cost-effective and ready for use anytime, the system is also free of flammable or irritating chemicals and ideal for disinfecting elderly homes.
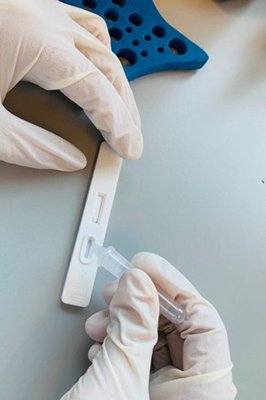
The University of Hong Kong is another public organisation participating in the scheme, including the trial of a COVID-19 diagnostic kit developed by ImmunoDiagnostics Ltd.
The kit, with a short turnaround time, can assist organisations such as medical institutions and testing laboratories in conducting rapid COVID-19 testing.
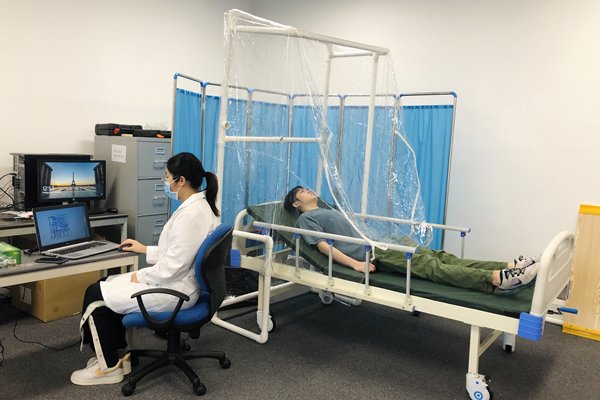
In addition, the university's School of Nursing has taken part in the trial project of a fast-track vented enclosure developed by City University.
Made of breathable protective materials, the enclosure aims to prevent viruses from spreading through the air in hospitals, thereby minimising the possibility of cross-infection between medical staff and patients.
The Innovation & Technology Commission said it will follow up on the progress of the projects for early realisation and commercialisation of the R&D outcomes to bring about anti-epidemic benefits for the community.
Click here for details.