Drug recall:
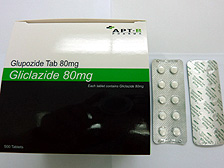
The Department of Health has instructed APT Pharma to recall a batch of Glupozide 80mg tablets (lot number: H00406) with the registration number HK-48195.
Glupozide 80mg tablets recalled
November 28, 2011
The Department of Health today instructed APT Pharma to recall a batch of Glupozide 80mg tablets (lot number: H00406) with the registration number HK-48195, after a plastic fragment was found embedded in one of the tablets.
The department said it is an isolated detection, with no indication other batches are affected.
The affected batch was manufactured in Guangdong in June. After packaging here, a total of 2,255 boxes of 500 tablets were supplied to various Hospital Authority hospitals.
Glupozide 80mg tablets, containing gliclazide, are used for the control of blood glucose in diabetic patients. The tablets require a doctor’s prescription and can only be sold in a pharmacy under the supervision of a pharmacist.
APT has set up a hotline 2341 7878 for enquiries.