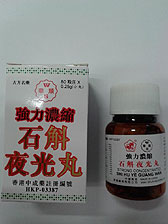
Bad batch:
The Department of Health has ordered the recall of [W.S.] Shi Hu Ye Guang Wan (Ye Guang Wan) as it contains excessive lead.
Bad batch:
The Department of Health has ordered the recall of [W.S.] Shi Hu Ye Guang Wan (Ye Guang Wan) as it contains excessive lead.
Chinese medicine recalled
November 08, 2012
The Department of Health today ordered manufacturer Wah Shun Medicine to recall a batch of its proprietary Chinese medicine called [W.S.] Shi Hu Ye Guang Wan (Ye Guang Wan) (registration number: HKP-03387, and batch number: 8011 WP1) as it contains excessive lead.
The Government Laboratory found a sample of the medicine contained about 1.3 times the permitted limit of lead.
Another medicine Ming Yan Pills (batch number: 8011 NY1) was also recalled as a precaution.
Both are for improving vision in adults. Prolonged exposure to excessive lead can cause anaemia and organ damage.
The manufacturer has set up a hotline (2571 0775) for enquiries.